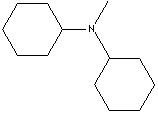
| N-METHYL DICYCLOHEXYLAMINE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 7560-83-0 |
|
| EINECS NO. | 231-453-4 | |
| FORMULA | (C6H11)2NCH3 | |
| MOL WT. | 195.34 | |
| H.S. CODE | ||
|
TOXICITY |
||
| SYNONYMS | Methyl Dicyclohexylamine; N-Cyclohexyl-N-methyl-cyclohexanamine; | |
| N,N-Dicyclohexylmethylamine; Dicyclohexylmethylamine; N-Ciclohexil-N-metilciclohexilamina; N-Cyclohexyl-N-méthylcyclohexylamine; | ||
|
DERIVATION |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear to yellow liquid | |
| MELTING POINT | ||
| BOILING POINT |
265 C | |
| SPECIFIC GRAVITY |
0.912 | |
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY | ||
| AUTOIGNITION |
| |
| NFPA RATINGS | ||
| REFRACTIVE INDEX |
| |
| FLASH POINT |
110 C | |
| STABILITY | Stable under ordinary conditions. | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Cyclohexane is the six-membered alicyclic hydrocarbon consisting of six carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms, C6H12. A cyclic compound is an organic compound that contains one or more closed rings of carbon atoms. The term alicyclic refers to cyclic compound that behaves chemically like aliphatic compounds (open-chain), which means the exclusion of carbocyclic compounds of aromatic rings with an array of ��-electrons characteristic. Cyclohexane is a colorless, highly flammable, mobile liquid with a pungent odor. It is insoluble in water and soluble in alcohol, ether, and almost organic solvents. Cyclohexane is a non-corrosive and quick volatilize liquid and sublimes between -5 to 5 C. Cyclohexane can exist in a number of interconvertible three-dimensional conformations, the two simplest being are the chair and boat conformation and others include the half-chair and twist-chair conformation. Cyclohexane can cause irritation to the eyes and mucous membranes in workers. Repeated and prolonged contact with skin may cause dermatitis. The substance has not been shown to cause the hematologic changes associated with exposure to benzene. Cyclohexane occurs naturally in crude oils. Some cyclohexane is recovered from petroleum streams by fractionation. The bulky commercial production of cyclohexane is based on hydrogenation of benzene in closed system. Cyclohexane consumption is linked almost entirely to nylon production. Nylon is further processed into fibers for applications in carpeting, automobile tire cord, clothing, and other growing industrial fields. Cyclohexane is used as a solvent, oil extractant, paint and varnish remover, dry cleaning material, and in solid fuels. It has been used as a insecticide itself. Cyclohexane is used as a chemical intermediate to produce target molecules. Natural compounds of one to five alicyclic rings with great variety and complexity are found particularly in steroids and terpenes. Cyclohexane structure, six membered-ring, is one of the major skeleton in nature. Cyclohexane derivatives can be used for the synthesis of pharmaceuticals, dyes, herbicides, plant growth regulator, plasticizers, rubber chemicals, cycloamines and other organic compounds. Dicyclohexylamine or a derivative thereof, is used as an additive for prevention of CO2, corrosion in steam pipelines and boilers. It is used as an intermediate for the production of antioxidants and vulcanization accelerator. It is used as a catalyst for flexible polyurethane foams. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear to yellow liquid | |
| PURITY |
99.0% min | |
|
FREE AMINE |
0.1% max | |
| WATER |
0.5% max | |
| TRANSPORTATION | ||
| PACKING | 180kgs in drum | |
| HAZARD CLASS | 8 (Packing Group: II) | |
| UN NO. | 2922 | |
| OTHER INFORMATION | ||
| Hazard Symbols: C, Risk Phrases: 22-34, Safety Phrases: 26-36/37/39-45 | ||